Lively exchange of latest knowledge and ideas
Plenary speaker: Ifan Stephens | Imperial college London
Ifan Stephens illustrated how electrocatalysis and battery research mutually inform each other. ‘There is a growing need for an electrochemical alternative to the Haber-Bosch process. Yet, at the moment, there is no catalyst that works well enough. Even though we have made a lot of progress to date, a lot still needs to be done,’ he stated. Since there seems to be an advantageous role for lithium to improve the selectivity of electrochemical processes, Stephens sought collaborations with battery researchers to understand the role of lithium in electrochemistry. He is now working on batteries himself, probing degradation mechanisms and studying their energy efficiency and lifespan.

Session 1: Water electrolysis - low temperature
Researchers presented their recent work on Using operando X-ray photoelectron spectroscopy and (soft) X-ray absorption spectroscopy methodology to study electrolyte effects and electrode restructuring; Understanding the role of nitrogen precursors in the composition and performance of platinum-free catalyst layers for polymer electrolyte fuel cells; Electrochemical oxidation of platinum and its hindering effect on the oxygen reduction reaction; High-tech manufacturing for high-performing, scalable, and cost-effective electrolysis; and How scanning electrochemical cell microscopy enables the evaluation of oxygen evolution reactions catalysts at the nanoscale.

Session 2: (Bio) CO2 electrochemical conversions 1
How to convert CO2 into valuable chemicals and fuels was the central theme in this session. The speakers provided an extensive overview of work on The molecular enhancement of heterogeneous electrolysis in CO2 conversion; Using AFM measurements to visualize dissolution-redeposition processes at copper-electrolyte interfaces during CO2 reduction; The production of adipic acid from CO2 and 1,3-butadiene; Bridging Fundamental In Situ Spectroscopy with Industrial Catalyst and Methods for CO2 electroreduction; and Insights into the electrochemical reduction of CO2 to formate via in-situ infrared spectroscopy.
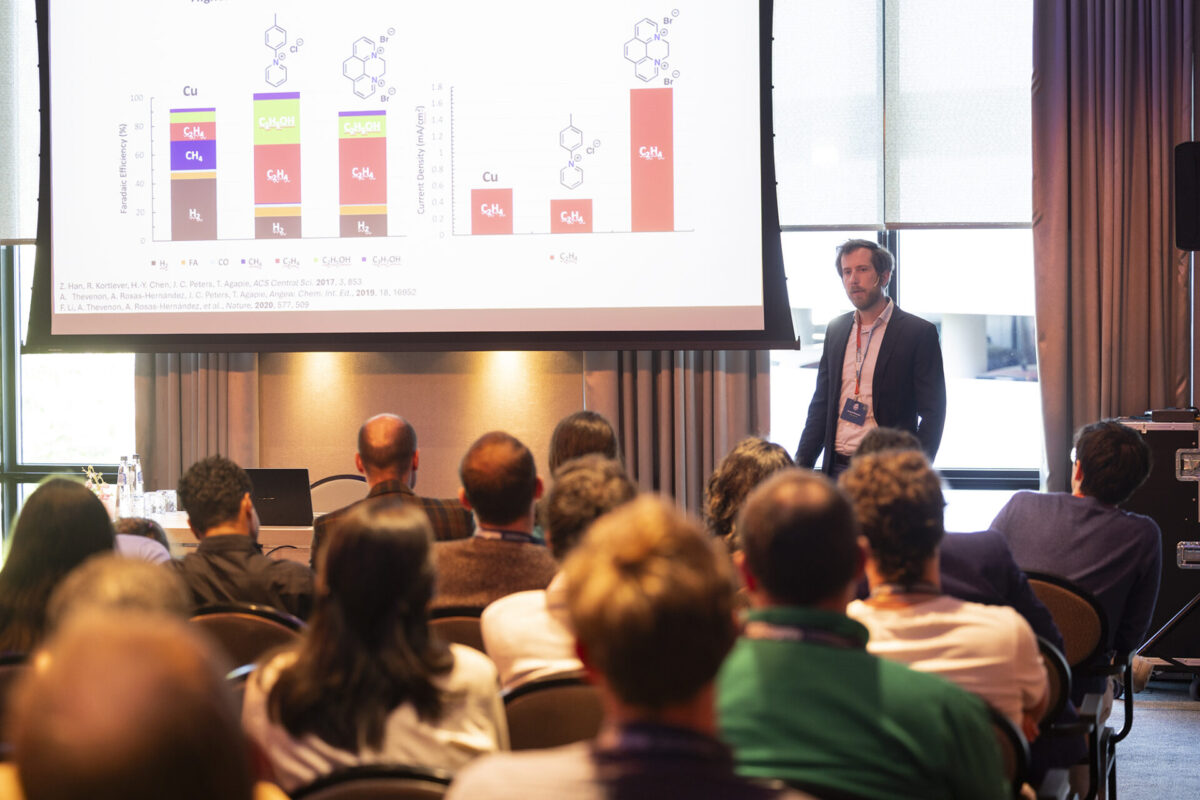
Session 3: Electrochemical energy storage
To bring the audience up to speed with the latest in battery research, the speakers delved into Interface engineering by atomic layer deposition for next-generation Li-ion batteries; Visualization of reactions in all-iron redox flow batteries via polarized neutron radiography; Interface engineering with spatial atomic layer deposition to improve the cycling stability of high-voltage cathodes for lithium-ion batteries; The development of high-performance bipolar membranes to enhance the energy efficiency of electrochemical regeneration steps; and Coupling benchtop NMR and EPR for characterizing redox flow batteries.

Plenary speaker: Marnix Wagemaker | Technical University of Delft
‘I want to give you a flavor of the typical challenges battery researchers encounter,’ Marnix Wagemaker introduced his talk. He spoke about the 99,999 percent challenge, referring to the strive for batteries that loose only 0,001 percent of their electrons per cycle. Besides having an increased lifespan, next generation batteries also need to be safer, cheaper, have a lower environmental impact, and consist of less rare materials. Going into the two major failure mechanisms for batteries, being electrolyte decomposition and mechanochemical failure, Wagemaker illustrated with some of his own groups’ recent research results the complexity of searching for new battery materials.

Session 4: Water electrolysis - high temperature
The second session diving into water electrolysis contained a mixture of high temperature and low temperate applications. Topics of discussion included: Materials and hybrid approaches to unlock the potential of SOECs and PCECs; An integral analysis of the faradaic efficiency and pressure drop in alkaline electrolysis to study shunt current; Techno-enviro-economic optimization of solid oxide electrolysis architectures; The influence of iron on AEM electrolysis; and How chemical storage has potential beyond the known chemical industry.

Session 5: (Bio) CO2 electrochemical conversions 2
The afternoon session on CO2 conversions delved further into the challenges of turning waste gas into useful products, via presentations on the following topics: Exploring the electrochemical reduction of CO2 on copper electrodes in organic solvent; Micro-scale modeling and process-limiting steps for Microbial Electrosynthesis (MES) for CO2 conversion; Operando benchtop NMR quantifies carbonation, water crossover and liquid products for high-current eCO2RR ; The Olympic challenge of reverse bias bipolar membranes in CO2 electrolysis; and Challenges of using impure feeds for CO2 electrolysis.

Watch the film of the day, May 12th 2025

Session 6: Electrochemical production of NH3, C-N and specialty chemicals
What kinds of chemical compounds can be produced via electrochemical routes, and how can we do that in an energy and cost efficient way? That was the theme central to this session, which contained presentations about: Computations for electrochemical production of NH3, C-N and specialty chemicals; Co-reduction of carbon dioxide and nitrate for electrosynthesis of urea; Adlayer formation in the double layer region of Au(111) in acetonitrile-based electrolytes; Electrochemical reduction of aldehydes as a pathway to formose-like reactions; and Electrooxidation of alcohols in hybrid water electrolysis.

Panel discussion
Moderated by Mark Boneschanscher, panel members Earl Goetheer, Martijn de Graaff, Andrea Ramirez Ramirez and Moniek Tromp discussed what the Dutch chemical industry will look like in 2040. If anything, not the same as today, the panelists were convinced. Energy intensive industries may have left the country, we might import our chemical compounds and have transformed toward offering specialty services only, and electrochemistry will probably have replaced a lot of the current fossil based processes. But, since 2040 is tomorrow, above all it is speed that we need to achieve this much needed transformation, they concluded.

Poster prizes
During ECNS 2025, the whopping amount of 74 posters were presented by PhD researchers and postdocs. Well-deserved poster prizes were awarded to Nina Chen (UvA, 3rd place), Mert Can Erer (TU/e, 2nd), and Mariangela Biggiero (UU, 1st place). Congratulations!

The journey will continue
Next year, the third ECNS will be organized by GroenvermogenNL, the University of Utrecht/Debye Institute and ANION.